A Parent’s Role in Promoting Survival
Intergenerational epigenetic inheritance and adaptations for parenthood and survival
Biological responses to physical, emotional, or environmental stress in a parent can be passed down to their offspring via germline DNA. This phenomenon, known as “transgenerational epigenetic inheritance,” presents a pathway that helps offspring learn about their parents’ environment without direct experience. Transgenerational epigenetic inheritance can cause changes in the brain, and suggests a mechanism by which parents could unknowingly, but adaptively, prepare future generations for the pressures that they have experienced.
While these adaptations could be advantageous if children are faced with stress or trauma similar to those that the parent experienced, they could prove harmful if such pressures are no longer present— causing a hyper stress-sensitive brain and an increased likelihood of serious mental illness. For example, following the Dutch Hunger Winter famine of 1944-1945, children of famine survivors had higher rates of death and illnesses such as diabetes, cardiovascular disease, anxiety, depression, and schizophrenia.
The Marlin Lab studies the behavioral and circuit-level brain changes that occur when an organism becomes a parent, and how life experiences can be passed down biologically to offspring. We utilize a combination of neural imaging, behavior, and molecular genetics to study the rodent olfactory and auditory systems, circuits crucial for environmental stressor detection. By asking how stress is transmitted across generations, we hope to uncover the driving force behind it, stop the psychological maladaptation it causes, and ultimately make breakthroughs in human mental health care.
Research Topic 1: The Parental Brain
Research Topic 2: Transgenerational Epigenetic Inheritance
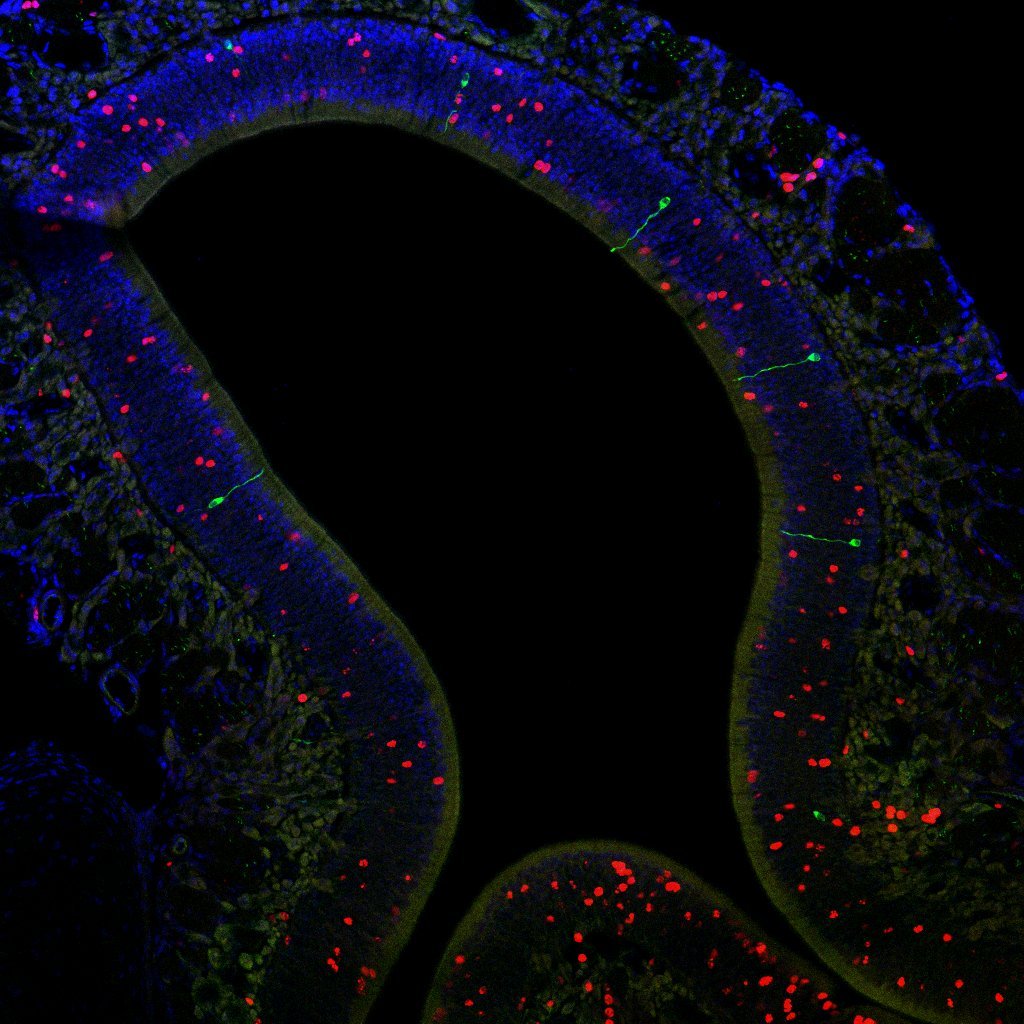
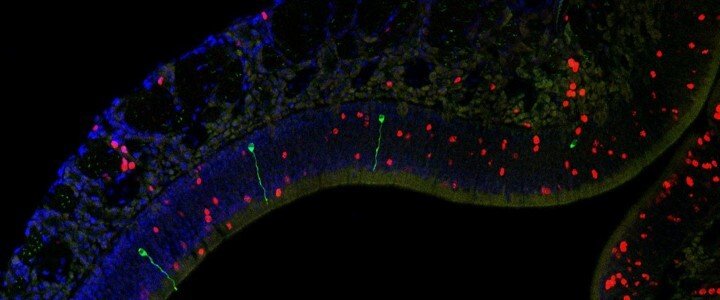
Research Topic 1: The Parental Brain
Investigating how the experience of parenthood itself changes the brain
Stress and trauma are not the only factors that can change our brains. Life events like parenthood can also produce changes in the brain to prepare parents in helping their offspring survive. In this project, our goal is to study the changes that parenthood and parenting behaviors induce in the brain, with a specific focus on the sensory circuits of hearing and smell.
-
Previous work with the Froemke Lab found that motherhood changes the auditory cortex through release of the hormone oxytocin so that mothers are more responsive to pup sounds and calls for help. In this aim, we are using specially designed mouse fMRI (created by collaborator Itamar Kahn) to evaluate which other brain regions are involved in auditory plasticity activated by parenthood. Prior work shows that mice that have not given birth can learn maternal behaviors through social examples, but how do changes in their brains compare to changes in the brains of parents? By investigating how parenthood itself changes the brain, we hope to understand which changes in the parental brain can be learned, and which are biologically activated by the process of producing offspring.
-
The Marlin Lab is also interested in understanding how parenthood changes the olfactory system. In this aim, we are investigating the changes that occur in the nose of rodent mothers before, during, and after pregnancy. We will also evaluate how changes in the mother’s nose compare to olfactory circuits in fathers and females who have not reproduced. Does the nose become more sensitive to the smell of a pup? If so, when does that change occur? Our goal is to understand how the olfactory circuit changes to prepare parents to take care of their offspring.

Research Topic 2: Intergenerational Epigenetic Inheritance
Using sensory circuits to test how parental experience is passed down to offspring
Our brains are wired to learn about potentially harmful elements in the environment. By quickly detecting and avoiding features of the world associated with danger, our brains keep us safe from harm and promote survival. In this project, our goal is to identify how stress-induced changes in the parental brain are biologically passed down to their offspring. But to understand how stress is passed between generations, we must first identify how the adult brain is altered by stress and the neural mechanisms underlying such changes.
-
We are investigating precisely when and how learning changes the sensory circuits in the adult brain, focusing on the rodent olfactory system during fear conditioning. In these experiments, mice are exposed to a smell (e.g., almond scent) that is simultaneously paired with a mild foot shock. Over time, mice learn that the smell predicts the occurrence of shock, and if given the opportunity, will engage in escape-related behaviors to avoid the aversive stimulus. Our goal is to build a precise timeline of changes that occur in the olfactory circuit over the course of several weeks of fear learning to better understand how it is represented in the brain. We also evaluate the extent to which fear learning can alter the olfactory circuit before the circuit no longer responds to the stressor. Finally, we test which brain regions and signaling factors are necessary to detect the coincidence of a sensation (e.g., a specific smell) and an environmental stressor (e.g., a shock) and link them in the brain.
-
Revealing how and when changes occur in the adult brain provides a road map to investigate how changes are perpetuated in their offspring’s neural landscape. In this aim, we examine olfactory development and function in mice with or without a parental history of stress. As with the adult brain, one goal is to discover the extent to which offspring circuits can be modified by stress, whether it be their own or inherited. For example, if offspring of stressed parents are exposed to the same smell/shock combination, are stress-induced neural changes amplified? Furthermore, are stress-induced changes in offspring neural circuitry permanent, or can inherited stress be unlearned? Finally, we are investigating how long stress-induced neural changes persist from parent to offspring by assessing the olfactory circuits of successive litters of stressed parents.
Tools & Techniques

Transcriptomics

Histology
- Brain clearing / iDisco
- Immunohistochemistry
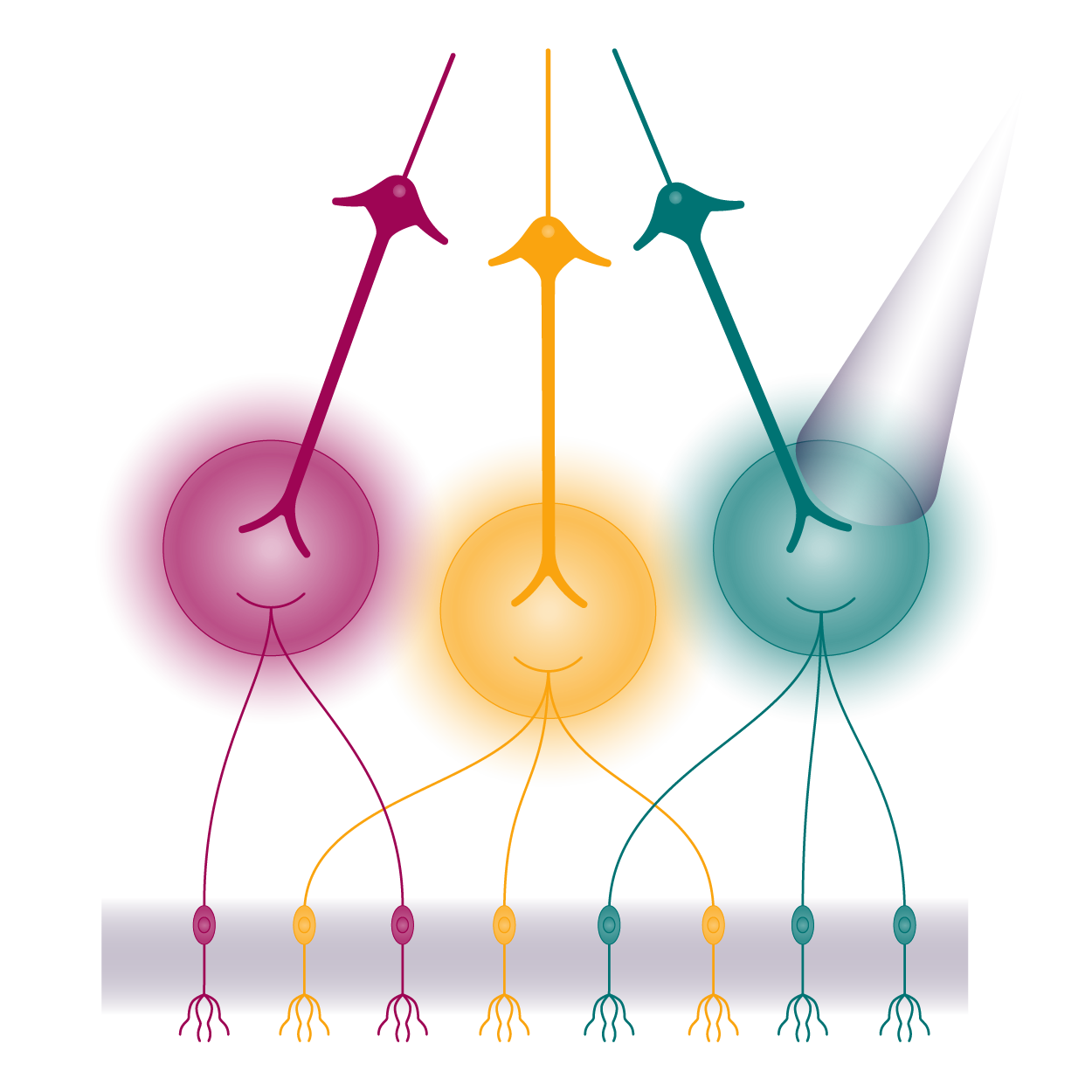
Circuit Monitoring & Manipulation
- fMRI
- Electrophysiology
- Calcium imaging
- DREADDS
- Optogenetics